Veins of Galen malformations (VOGMs) belong to a category of disorders referred to as cerebral vascular malformations, impacting the blood vessels within the brain. Within this abnormality, multiple arteries in the brain connect directly to the median prosencephalic vein without an intervening capillary bed. The elevated pressure throughout the veins of the brain can result in pulmonary hypertension, brain damage, and congestive heart failure, frequently occurring shortly after birth. A new study led by WashU graduate student Shujuan Zhao, co-mentored by Dr. Sheng Chih (Peter) Jin, Assistant Professor of Genetics and Pediatrics, Washington University School of Medicine, and Dr. Kristopher T. Kahle, Director of Pediatric Neurosurgery, Massachusetts General Hospital, sheds light on new genetic mutations related to the disease and validations of the identified genes in mouse and zebrafish models. This collaborative work was recently published in Nature Communications.
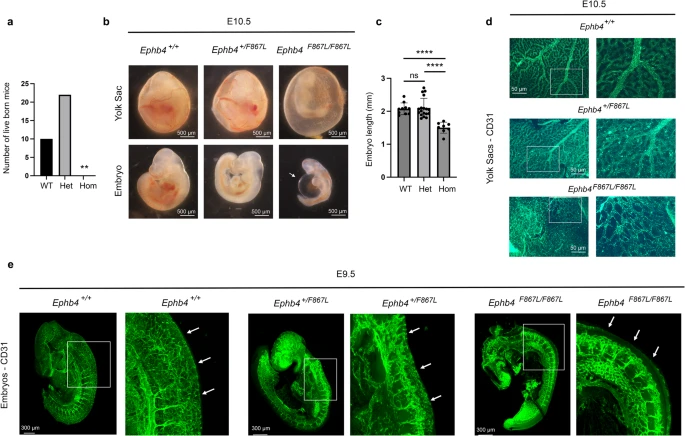
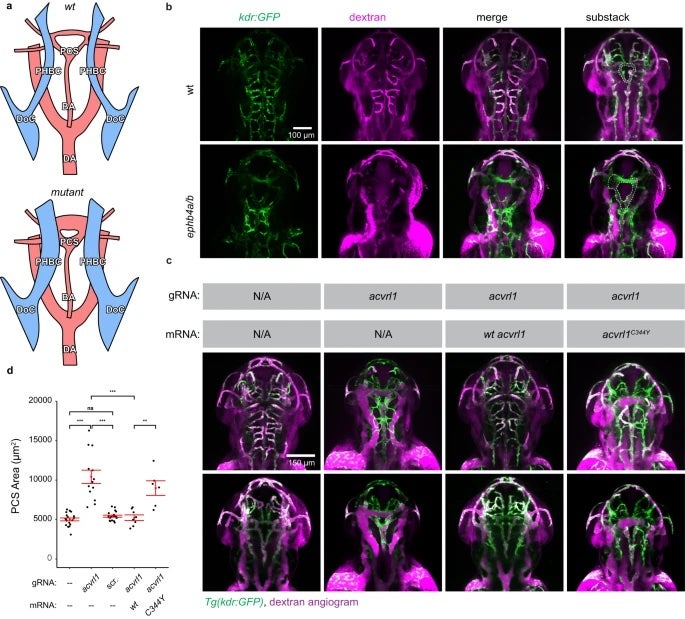
Studying rare diseases is no easy task. One of the biggest challenges faced by the researchers is collecting patient samples. In this paper, a total of 114 cases were collected, the world’s largest VOGM cohort to date. With an estimated incidence of one in 30,000, very few studies of VOGM have been published so far. The last paper co-authored by Dr. Sheng Chih (Peter) Jin published in 2018 studying this disease had a cohort of 55 cases.
Initiated over five years ago during Dr. Jin’s postdoc training at Yale, the study was supported by his postdoc mentor Dr. Richard P. Lifton, the then Chair of the Department of Genetics at Yale School of Medicine. Shujuan Zhao, a graduate student in Dr. Jin’s lab at Washington University School of Medicine, started leading this research project in 2020. “Previously, the research only reported potential causal genes, but they were not validated experimentally. For this research, it’s very significant that we included functional studies. We validated the genes in vivo and in vitro.” said Shujuan Zhao, the first author of the paper. “We found several de novo variants linked to the MAPK signaling pathway, strongly suggesting that this pathway may be causal and further validated by our functional experiments. This can be a potential therapeutic strategy for treatment of the disease.”
The mutations identified in this research underwent validation in mouse and zebrafish models with mouse experiments mostly completed at University of Michigan-Ann Arbor and zebrafish experiments done at the Zebrafish Core at Yale. In addition to whole-exome sequencing data, the research team also included human cerebrovascular single-cell sequencing data in the analysis which led to the proposal of a new molecular and cellular mechanism underlying the disease.
“In this study, we used a mix of genome sequencing and single-cell transcriptomic study, incorporating functional genomics to help us get a more complete picture of the disease mechanism.”
Dr. Sheng Chih (Peter) Jin
However, there is still more to learn about VOGM. Potential disease-causing genes were only found in about 15% of the patients. Jin said, that means more than 80% of patients still await an explanation. Given the disease’s high heterogeneity, further efforts are needed to enhance diagnostic solutions for these patients.
Featured in this article
Shujuan Zhao

Shujuan studied Pharmaceutical Engineering at Central South University for her undergraduate degree with a keen interest in developing drugs to treat diseases. She then became a research assistant in the Shanghai Institute of Organic Chemistry where she acquired a solid foundation of organic chemistry, developed synthetic methods for a few novel molecules that did not exist before. As a PhD student at Washington University School of Medicine, Shujuan wanted to understand how human diseases develop which led her to study human genetics and the underlying molecular mechanisms causing a disease.
Jin Lab

The Jin Lab is dedicated to developing innovative computational methods and tools for investigating complex variants underlying rare diseases. Leveraging various genomic technologies and functional genomics, the Jin Lab identifies gene mutations and subsequently delves into the mechanisms of the diseases. Through the integration of diverse omics data types and epigenetic functional annotations, the lab conducts integrative genomic analyses to gain a deeper understanding of the molecular basis of cardiovascular diseases and neurological disorders. Collaborating with clinicians, the Peripheral Neuropathy Research Registry, the Cerebral Palsy Research Network, and the WashU Undiagnosed Diseases Network, the lab assembles thoroughly phenotyped cohorts for gene discovery.