A collaborative research effort between laboratories at Washington University School of Medicine has identified molecular and cellular mechanisms by which a ketogenic diet (KD) alters synaptic communication in the brain — revealing how metabolic state can reshape neuronal networks at the transcriptional, epigenetic, and physiological levels. Spearheaded by first authors Marion Stunault, PhD, Pan-Yue Deng, PhD and Anjali Yadav, PhD, the study, “Ketogenic diet dampens excitatory neurotransmission by shrinking synaptic vesicle pools,” was recently published in Cell Reports and provides foundational insight into how diet-induced metabolic changes influence excitatory circuits in the hippocampus.
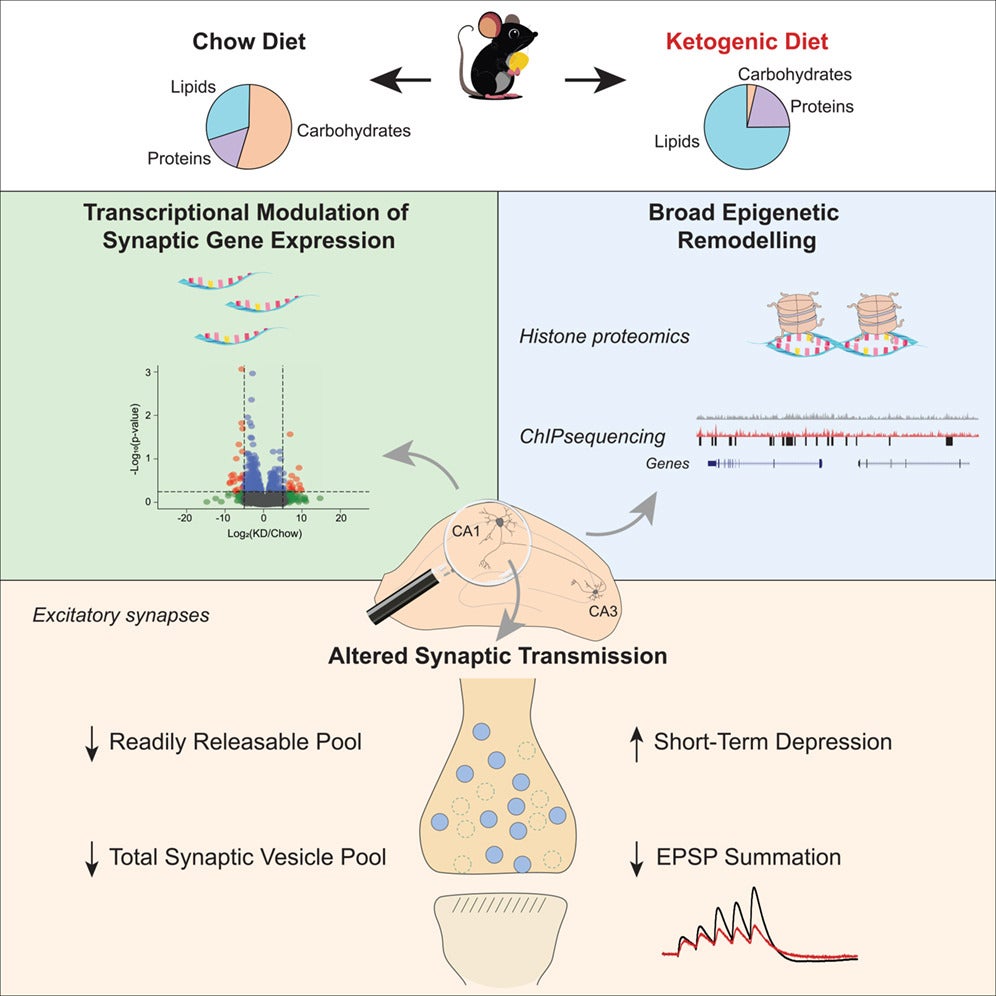
Hippocampal synapses — critical for learning, memory, and regulation of neuronal excitability — require precise coordination of neurotransmitter release and gene expression. Although ketogenic diets have long been used clinically to treat drug-resistant epilepsy, the cellular mechanisms underlying their neuroprotective effects have remained incompletely understood. To address this question, the research team combined transcriptomics, epigenomic profiling, electrophysiology, and ultrastructural analysis to determine how KD reshapes synaptic biology at multiple levels of organization.
The study was led by senior authors Ghazaleh Ashrafi, PhD, Associate Professor of Cell Biology & Physiology, Vitaly A. Klyachko, PhD, Professor of Cell Biology & Physiology and Gabor Egervari, MD, PhD, Assistant Professor of Genetics and Biochemistry. This ambitious project explored the effect of KD on the hippocampus at key molecular and functional levels, requiring close collaboration among three multidisciplinary labs. The complementary expertise of the team enabled investigation of the diet’s effects on hippocampal gene expression and synaptic morphology (Ashrafi lab), the epigenome (Egervari lab), as well as synaptic transmission itself (Klyachko lab), underscoring the strength of cross-disciplinary research within the Department of Genetics and across Washington University.
The researchers found that a ketogenic diet induces broad transcriptional and epigenetic remodeling within the hippocampus, reshaping gene expression programs that regulate excitatory synaptic function. These molecular changes translate into measurable physiological effects: electrophysiological recordings demonstrated reduced short-term plasticity and decreased synaptic gain at excitatory CA3–CA1 synapses, indicating dampened excitatory neurotransmission. Ultrastructural analyses further revealed that ketogenic diet feeding reduces both the readily releasable pool and the total pool of synaptic vesicles, providing a structural basis for diminished synaptic output. Together, these findings establish a mechanistic link between metabolic state, chromatin regulation, and functional remodeling of neuronal circuits.
By defining how a metabolic intervention propagates from gene regulation to synaptic physiology, this work advances understanding of the molecular logic underlying ketogenic diet therapy. The findings may inform new strategies aimed at modulating excitatory/inhibitory balance in epilepsy and other neurological disorders characterized by circuit hyperexcitability.